


Prix FOB
Obtenir le dernier prix( Negotiable )
|50000 Piece Minimum Order
Pays:
Others1
N ° de modèle:
11901
Prix FOB:
( Negotiable ) Obtenir le dernier prix
Localité:
Others1
Prix de commande minimale:
-
Commande minimale:
50000 Piece
Packaging Detail:
as per attach photo or brochure
Heure de livraison:
As per cutomer requirements
Capacité de Fournir:
300000 Piece per Day
Payment Type:
T/T
Groupe de produits :
Personne à contacter Mr. Sam
Room 302C, No.718, Blv San Yuan Li, Bai Yuan District,, Changping, Guangdong
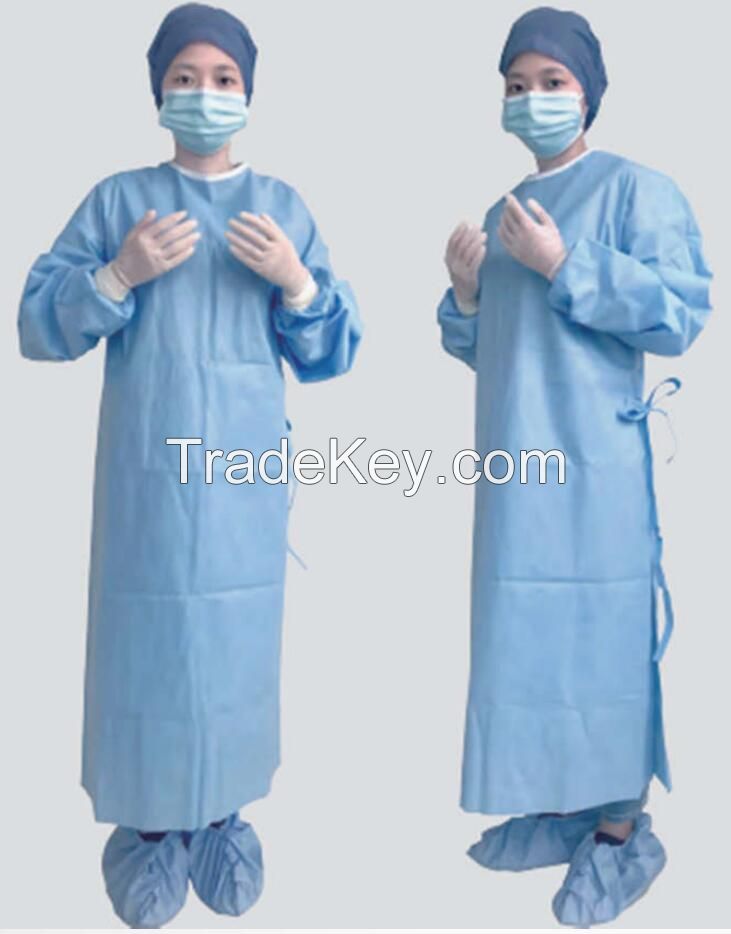
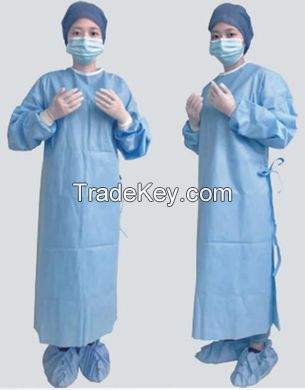
Product
Name: Sterile
Surgical Suit
Model No.:According
to Customer Specification
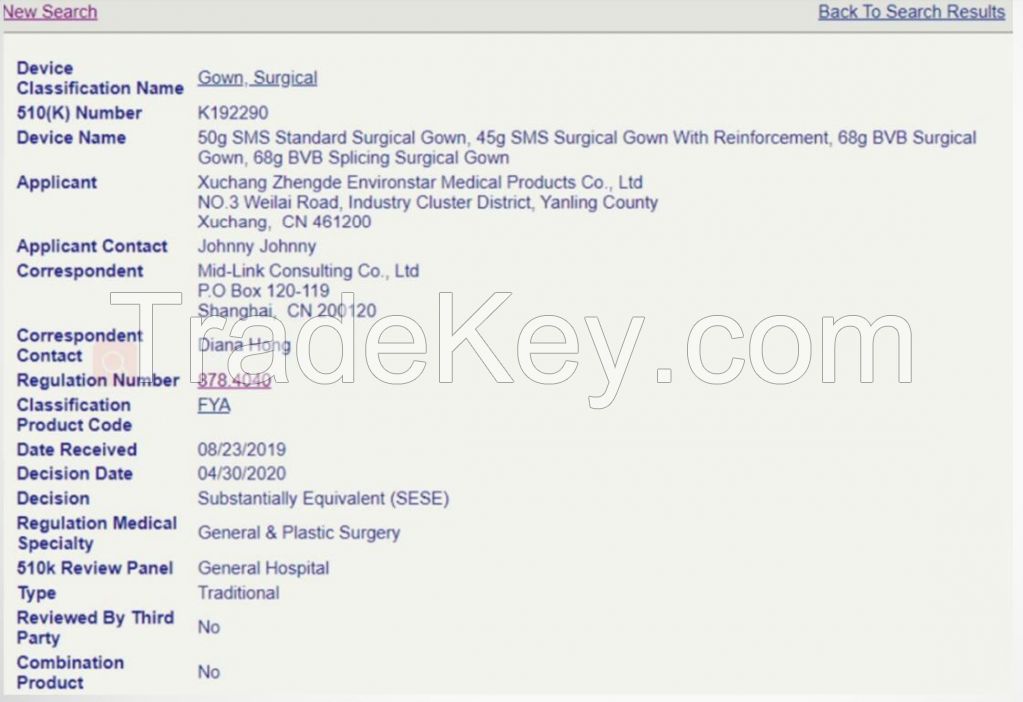
Certificate:
510K No.: K192290
TUV CE Based on EN
13795-1:2019 (CE
No.: G2S
073966 0008 Rev. 01)
TUV Level 4 Test Report from TUV, Based on
AAMI PB70:2012
TUV Level 3 Test Report Based on AAMI
PB70:2012
TUV Level 2 Test Report Based on AAMI
PB70:2012
TUV
ISO 13485
We herewith declare that the
above mentioned products meet the transposition into national law,
the provisions of the following EC Council directives and
Standards. All supporting documentations are retained under the
premises of the manufacturer.
UMDNS
Code:11901
Classification (MDD Annex
IX): I
Sterile, Rule 1
Conformity Assessment
Route:Annex
V section 3
General applicable
directives:
Medical Device Directive: COUNCIL
DIRECTIVE 93/42/EEC
Standard
Applied:
EN ISO 15223-1:2016
EN1041:2008 EN ISO
13485:2016
EN ISO 14971:2012
EN 13795:2011
Notified
Body:TV
SD Products Service GmbH, Ridlestr. 65, 80339, Munchen,
Germany
Identification
Number:0123
(EC)
Certificate(S):G2S
073966 0008 Rev.01
Expire Date of the
Certificate:2024-05-26
Start of CE
Marking:2010-11-26
Place,Date of
Issue:Xuchang,
2019-10-15
*Ergonomic fit, enables freedom
of movement
*Reinforced
*Breathable and comfortable,
suitable for most procedures
*Specially treated to repel
low-tension surface fluids
Manufacturing
Center:10
Sterilization Center:
2
Employee:
4000
RD
Center:2
Market: 74
Countries
| Pays: | Others1 |
| N ° de modèle: | 11901 |
| Prix FOB: | ( Negotiable ) Obtenir le dernier prix |
| Localité: | Others1 |
| Prix de commande minimale: | - |
| Commande minimale: | 50000 Piece |
| Packaging Detail: | as per attach photo or brochure |
| Heure de livraison: | As per cutomer requirements |
| Capacité de Fournir: | 300000 Piece per Day |
| Payment Type: | T/T |
| Groupe de produits : | surgical suit |